Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
*; Mihara, Morihiro;
JNC TN8430 2001-007, 56 Pages, 2002/01
In the geological disposal concept of radioactive wastes, a kind of clay with sorption ability and low permeability, called bentonite, is envisaged as an engineered barrier system in the geological repository. Also, the cemetitious material is envisaged as the backfill material in the vaults and the structure material of the vaults. The groundwater in contact with the cementitious material will promote hyperalkaline conditions in the repository environment and these conditions will affect the performance of the bentonite. Therefore, it is necessary to investigate the interaction between the cementitious material and the bentonite for the evaluation of long term stability of the disposal system. In this study, for the identification and the investigation of the secondary minerals, the batch immersion experiments of the powder bentonite were carried out using synthetic cement leachates (pH=7, 12.5, 14) at 200 C. As the results, it was confirmed that Na as exchangeable cations in the bentonite can exchange relatively easily with Ca in the solution from the experiment results. And the ratio of cation exchange was estimated to be about 25% based on the amount of exchangeable cations Ca
C. As the results, it was confirmed that Na as exchangeable cations in the bentonite can exchange relatively easily with Ca in the solution from the experiment results. And the ratio of cation exchange was estimated to be about 25% based on the amount of exchangeable cations Ca between layers. Furthermore, it was concretely shown that the generation of analcime might be affected by the Na concentration from results of the solution analyses and a stability analysis of analcime using the chemical equilibrium model, in addition to the pH in the solution.
between layers. Furthermore, it was concretely shown that the generation of analcime might be affected by the Na concentration from results of the solution analyses and a stability analysis of analcime using the chemical equilibrium model, in addition to the pH in the solution.
; ; ; ; ; *; *
JNC TN8400 2001-026, 29 Pages, 2001/12
The measurement condition by spectrophotometry was evaluated to measure Np content in MOX fuel containing Np. The Np concentration was obtained by measuring the 727nm absorption peak, after the valence of Np in the sample solution was adjusted to the Np(Ⅳ). The calibration curve showed the linearity up to Np concentration 0.8mg/ml. Though Pu and U quantity were respectively added to the Np solution to 30 times and 60 times of Np concentration, there was no effect to the Np analysis. By using this method, relative standard deviation (RSD) of the analyzed values of Np content for 2%Np - MOX fuel was about 4%. In addition, It was confirmed that the Np content could be measured without separating Np from Pu and U. This method can be sufficiently applied as a quick simple method to analyze Np content in MOX fuel containing Np.
*;
JNC TN8430 2001-006, 65 Pages, 2001/10
We had been conducted to study hydraulic permeability along fracture intersection by NETBLOCK system using natural rock specimen. Since the permeability of this rock specimen fracture is high, it was suggest that turbulent flow might be occurred in available range of measurement system. In case of turbulent flow, estimated permeability and fracture aperture from test data tend to be low. Therefore we should achieve laminar flow. This study was used the high viscosity liquid instead of water, and test conditions which could attain laminar flow with the rock specimen was examined. The rock specimen is granite rock, has natural Y-type fractures intersection. A solution of Methyl-cellulose is used as high viscosity liquid. Due to the high viscosity liquid, hydraulic head could be measured in the wide range, and high viscosity liquid improved the accuracy of measurement. Laminar flow could be achieved in the rock specimen by the high viscosity liquid over 0.1wt%.
JNC TN8400 2001-008, 36 Pages, 2001/03
Research on geologic disposal of high-level radioactive waste(HLW) has been underway in many countries. Bentonite exhibiting a low permeability, high swelling property and high sorption capacity for many radioelements is proposed as a buffer material in many countlies. Recently, cementitious materials are considered as candidate matelials for the geologic disposal of high-level radioactive waste. As the pH and the Ca, Na, K contents of hyperalkaline pore water from the cementitious materials are high, this hyperalkaline pore water would alter the buffer material. The main aim of this study is to investigate the effect of alkaline pore water into the bentonite. Used materials are montmorillonite, albite and quartz composing bentonite. These minerals mixed in a constant ratio (1:1wt%) made to react to distilled water and the alkali solutions (pH11-13). These studies have been conducted at temperatures of 50 - 150 C and run times of 10 - 200 day. XRD(X-ray powder diffraction) and SEM (Scanning Electron Microscopy) analyses were applied to studying the structure and quantitative data of each sample. From the result of this study, the main formed mineral of this experiment was analcime, which showed the tendency with a large amount of generation at a higher pH and temperature. Quantitative data of this study was conducted by X-ray powder diffraction method. THe order of the amount of the second analcime in each experiment is shown in the following. Montmorillonite and albite mixing test
C and run times of 10 - 200 day. XRD(X-ray powder diffraction) and SEM (Scanning Electron Microscopy) analyses were applied to studying the structure and quantitative data of each sample. From the result of this study, the main formed mineral of this experiment was analcime, which showed the tendency with a large amount of generation at a higher pH and temperature. Quantitative data of this study was conducted by X-ray powder diffraction method. THe order of the amount of the second analcime in each experiment is shown in the following. Montmorillonite and albite mixing test  Montmorillonite test
Montmorillonite test  Montmorillonite and quartz mixing test Activation energies (E
Montmorillonite and quartz mixing test Activation energies (E ) using the quantitative data of each test are shown in the following. (1)Montmorillonite test : 54.9kJ/mol (2)Montmorillonite and albite mixing test : 51.9kJ/mol (3)Montmorillonite and quartz mixing test : 59.6kJ/mol
) using the quantitative data of each test are shown in the following. (1)Montmorillonite test : 54.9kJ/mol (2)Montmorillonite and albite mixing test : 51.9kJ/mol (3)Montmorillonite and quartz mixing test : 59.6kJ/mol
Kitamura, Akira; *
JNC TN8400 2001-009, 54 Pages, 2001/01
Spectroscopic measurements of neodymium(III) and samarium(III) were carried out by spectrophotometer and laser-induced photoacoustic spectroscopic (LPAS) system for the investigation of the detection limit of both systems. The absorption spectra and photoacoustic spectra of Nd and Sm
and Sm were obtained with varying the concentration of the ions from 2
were obtained with varying the concentration of the ions from 2 10
10 to 2
to 2 10
10 mol
mol dm
dm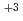 . The absorption spectrum of Nd
. The absorption spectrum of Nd was also determined by a special spectrophotometer, of which the measurement cell was set in a glove box filled with inert nitrogen gas. For the comparison with these photoacoustic and absorption spectra, the absorption spectra of Nd
was also determined by a special spectrophotometer, of which the measurement cell was set in a glove box filled with inert nitrogen gas. For the comparison with these photoacoustic and absorption spectra, the absorption spectra of Nd and Sm
and Sm were determined by an usual spectrophotometer with the light-path lengths of 1 cm and 10 cm. The detection limit of the photoacoustic measurement was reported much lower than that of absorbance measurement by several researchers. However, the present study was concluded that the detection limit of photoacoustic measurement with the present LPAS system was similar to that of absorbance measurement with the light-path length of 10 cm. The detection limits of neptunium(IV,V) were estimated and the possibility of the speciation of neptunium(IV) was discussed from the results of the present study.
were determined by an usual spectrophotometer with the light-path lengths of 1 cm and 10 cm. The detection limit of the photoacoustic measurement was reported much lower than that of absorbance measurement by several researchers. However, the present study was concluded that the detection limit of photoacoustic measurement with the present LPAS system was similar to that of absorbance measurement with the light-path length of 10 cm. The detection limits of neptunium(IV,V) were estimated and the possibility of the speciation of neptunium(IV) was discussed from the results of the present study.
Fukumoto, Masahiro; Nishikawa, Yoshiaki*; Kagawa, Akio; Kawamura, Kazuhiro
JNC TN8400 2001-002, 23 Pages, 2000/12
The soluble organic compounds generated by radiological degradation of asphalt ( ray) were confirmed as a part of influence of the bituminized waste degradation in the TRU waste repository. Especially, the influence of the nitrate was focused on. As a result, the concentration of the soluble organic compounds generated by radiological degradation of asphalt (10MGy,
ray) were confirmed as a part of influence of the bituminized waste degradation in the TRU waste repository. Especially, the influence of the nitrate was focused on. As a result, the concentration of the soluble organic compounds generated by radiological degradation of asphalt (10MGy,  ray which is correspond to absorbed dose of asphalt for 1,000,000 years) were lower (each formic acid : about 50mg/dm
ray which is correspond to absorbed dose of asphalt for 1,000,000 years) were lower (each formic acid : about 50mg/dm , acetic acid : about 30mg/dm
, acetic acid : about 30mg/dm and oxalic acid : about 2mg/dm
and oxalic acid : about 2mg/dm ) than that of the formic acid, the acetic acid and the oxalic acid which Valcke et al. had shown (the influence of the organic at the solubility examination which uses Pu and Am). Moreover, the change in the concentration of TOC and the soluble organic compounds (formic acid, acetic acid and oxalic acid) is little under the existence of nitrate ion. That is, the formic acid and acetic acid which can be organic ligands were generated little by oxidative decomposition of asphalt in the process that nitrate ion becomes nitrite ion by radiation. The influence of the soluble organic compounds by the radiological degradation of the asphalt (
) than that of the formic acid, the acetic acid and the oxalic acid which Valcke et al. had shown (the influence of the organic at the solubility examination which uses Pu and Am). Moreover, the change in the concentration of TOC and the soluble organic compounds (formic acid, acetic acid and oxalic acid) is little under the existence of nitrate ion. That is, the formic acid and acetic acid which can be organic ligands were generated little by oxidative decomposition of asphalt in the process that nitrate ion becomes nitrite ion by radiation. The influence of the soluble organic compounds by the radiological degradation of the asphalt ( ray) on adsorption and solubility by the complexation of radionuclides in the performance assessment can be limited.
ray) on adsorption and solubility by the complexation of radionuclides in the performance assessment can be limited.
 to obtain the high U solution
to obtain the high U solution; *; Sakurai, Koji*; ; Nomura, Kazunori; *
JNC TN8400 2000-032, 98 Pages, 2000/12
Concerning the preparation of high U solution for the crystallization process and the application of UO powder dissolution to that, the effects of final U concentration, dissolution temperature, nitric acid concentration and powder size on the dissolution of UO
powder dissolution to that, the effects of final U concentration, dissolution temperature, nitric acid concentration and powder size on the dissolution of UO powder in the nitric acid where the final U concentration was
powder in the nitric acid where the final U concentration was  800g/L were investigated. The experimental results showed that the solubility of UO
800g/L were investigated. The experimental results showed that the solubility of UO decreased with the increase of final UO
decreased with the increase of final UO concentration and powder size, and with the decrease of dissolution temperature and nitric acid concentration. It was also confirmed that in the condition where the final U concentration was sufficiently lower than the solubility of U, UO
concentration and powder size, and with the decrease of dissolution temperature and nitric acid concentration. It was also confirmed that in the condition where the final U concentration was sufficiently lower than the solubility of U, UO dissolution behavior in the high U solution could be estimated with the equation based on the fragmentation model which we had already reported. Based on these experimental results, the dissolution behavior of irradiated MOX fuel in high U solution was estimated and the possibility of supplying high U solution to the crystallization process was discussed. In the preparation of high U solution for the crystallization process, it was estimated that the present dissolution process (dissolution for fuel pieces of about 3cm long) needed a lot of time to obtain a high dissolution yield, but it was shorted drastically by the pulverization of fuel pieces. The burst of off-gas at the early in the dissolution of fuel powder seems to be avoidable with setting the appropriate dissolution condition, and it is important to optimize the dissolution condition with considering the capacity of off-gas treatment process.
dissolution behavior in the high U solution could be estimated with the equation based on the fragmentation model which we had already reported. Based on these experimental results, the dissolution behavior of irradiated MOX fuel in high U solution was estimated and the possibility of supplying high U solution to the crystallization process was discussed. In the preparation of high U solution for the crystallization process, it was estimated that the present dissolution process (dissolution for fuel pieces of about 3cm long) needed a lot of time to obtain a high dissolution yield, but it was shorted drastically by the pulverization of fuel pieces. The burst of off-gas at the early in the dissolution of fuel powder seems to be avoidable with setting the appropriate dissolution condition, and it is important to optimize the dissolution condition with considering the capacity of off-gas treatment process.
JNC TN8400 2000-030, 17 Pages, 2000/12
As a representative of natural marine groundwater, the author selected pumped water from a Quaternary sedimentary aquifer of the Mobara gas-field in Japan and measured the concentration of total organic carbon (TOC) and of organic acid anions (formic, acetic, lactic, succinic, humic, fulvic, propionic, valeric and butyric acids). The concentration of TOC ranged from 22 1 to 24
1 to 24 0mg/L. As organic acid anions, only succinic and fulvic acids were detected and each concentration was given to be from 5.8
0mg/L. As organic acid anions, only succinic and fulvic acids were detected and each concentration was given to be from 5.8 0.5 to 8.3
0.5 to 8.3 0.3 and from 3.3
0.3 and from 3.3 0.2 to 3.5
0.2 to 3.5 0.2mg/L, respectively. By consideration of the temperature and the [SO
0.2mg/L, respectively. By consideration of the temperature and the [SO
 ] of the groundwater, it is inferred that the organic acid has been significantly decomposed by activities of microbes, such as the fermentation process, CH
] of the groundwater, it is inferred that the organic acid has been significantly decomposed by activities of microbes, such as the fermentation process, CH COO
COO + H
+ H O = HCO
O = HCO
 + CH
+ CH .
.
Yamanaka, Shinsuke*; Abe, Kazuyuki
JNC TY9400 2000-004, 78 Pages, 2000/03
no abstracts in English
; Nishikawa, Yoshiaki*; Kagawa, Akio;
JNC TN8400 2000-017, 30 Pages, 2000/03
The influence of the cement additives on the distribution coefficients of americium-241 to the Ca-bentonite was confirmed. The adsorption experiment of americium-241 to Ca-bentonite with cement additives was performed by the batch method, as a part of the influence evaluation of the organic in the research of TRU waste disposal. As a result, the distribution coefficient of americium-241 to the Ca-bentonite was over 1.2E+3m /kg in the condition of the absence of cement additives. In the case of low concentration (0.3g/kg) of the naphthalenesulfonic acid type cement additives, the distribution coefficient was 5.2E+2m
/kg in the condition of the absence of cement additives. In the case of low concentration (0.3g/kg) of the naphthalenesulfonic acid type cement additives, the distribution coefficient was 5.2E+2m kg. And, in the case of high concentration (30g/kg) of the same cement additives, the distribution coefficients was 2.0E-1m
kg. And, in the case of high concentration (30g/kg) of the same cement additives, the distribution coefficients was 2.0E-1m /kg. On the other hand in the case of flow concentration (0.5g/kg) of the polycarboxylic acid type cement additives, the distribution coefficients was over 1.3E+3m
/kg. On the other hand in the case of flow concentration (0.5g/kg) of the polycarboxylic acid type cement additives, the distribution coefficients was over 1.3E+3m /kg. And, in the case of high concentration (50g/kg) of the same cement additives, the distribution coefficient was 1.8E-1m
/kg. And, in the case of high concentration (50g/kg) of the same cement additives, the distribution coefficient was 1.8E-1m /kg. Here, selected cement additives concentrations were based on a standard concentration of 10g/kg when the ratio of water:cement is about one. From these results, the distribution coefficient of americium-241 to the Ca-bentonite decreases according cement additive concentration. The distribution coefficients were similar on different kinds of cement additives. The cement additives concentration influences the distribution coefficient. The distribution coefficient was small in the case of high concentration of the cement additives. That is, it is thought that the cement additives have small influences on the distribution coefficient of americium-241 to the Ca-bentonite in the case of low concentration, though the cement additives have influences on the distribution coefficient of americium-241 to the Ca-bentonite in the case of high concentration.
/kg. Here, selected cement additives concentrations were based on a standard concentration of 10g/kg when the ratio of water:cement is about one. From these results, the distribution coefficient of americium-241 to the Ca-bentonite decreases according cement additive concentration. The distribution coefficients were similar on different kinds of cement additives. The cement additives concentration influences the distribution coefficient. The distribution coefficient was small in the case of high concentration of the cement additives. That is, it is thought that the cement additives have small influences on the distribution coefficient of americium-241 to the Ca-bentonite in the case of low concentration, though the cement additives have influences on the distribution coefficient of americium-241 to the Ca-bentonite in the case of high concentration.
Iwase, Masanori*
JNC TJ8400 2000-063, 78 Pages, 2000/03
This study is aimed at controlling oxidation reaction of molten metal by ash in incineration systems, and at positively utilizing the oxidation reaction for decontamination of slag. In this year, in order to investigate physico-chemical properties of mixed fused salt containing alkali sulfates, with special focus on the behaviour of oxygen anion in the melts, Cu / Cu
/ Cu redox equilibrium experiments were carried out. Among the effect of various parameters on Cu
redox equilibrium experiments were carried out. Among the effect of various parameters on Cu / Cu
/ Cu ratio in binary and ternary alkali sulfate melts, the effect of partial pressures of oxygen and SO
ratio in binary and ternary alkali sulfate melts, the effect of partial pressures of oxygen and SO was mainly investigated in the study. Variation in Cu
was mainly investigated in the study. Variation in Cu / Cu
/ Cu ratio were presented as the function of partial pressures of oxygen and SO
ratio were presented as the function of partial pressures of oxygen and SO , respectively. Possible thermodynamic interpretation were made on the experimental results. In addition, the dissolution of Cr
, respectively. Possible thermodynamic interpretation were made on the experimental results. In addition, the dissolution of Cr O
O in mixed alkali sulfates were also investigated as a first step to elucidate the mechanism of hot corrosion. With this investigation, an important finding was obtained that the solubility of Cr
in mixed alkali sulfates were also investigated as a first step to elucidate the mechanism of hot corrosion. With this investigation, an important finding was obtained that the solubility of Cr O
O for melts with same average ionic radius, in other words, oxygen ion activity, were essentially identical under constant temperature and atmosphere.
for melts with same average ionic radius, in other words, oxygen ion activity, were essentially identical under constant temperature and atmosphere.
*; *; *; *
JNC TJ8400 2000-061, 92 Pages, 2000/03
Crystallization procedure is considered to have an advantage in recovering rather pure uranium from contaminated uranium solution and to be applicable for a new reprocessing process. It was confirmed until last year that the reprocessing process with crystallization procedure has a sufficient advantage. But the data for Pu crystallization is very rare. although it is necessary for design of the process with crystallization procedure. In this study, a beaker scale plutonium test was performed in AEA Technology Harwell Laboratory to confirm a behavior of Pu (IV) nitrate under crystallization condition. The results were examined by Mitsubishi Materials Corporation. Test item was a measurement of temperature in case of Pu (IV) nitrate crystallization or freezing of the solution in the following six parameters. (Pu(g/L):200, 100, 50, HNO (m):6, Pu valence:4). (Pu(g/L):200, 100, 50, HNO
(m):6, Pu valence:4). (Pu(g/L):200, 100, 50, HNO (m):4, Pu valence:4). Test results were as follows. (1)Pu(IV) nitrate crystallization was not observed even in the case 200g Pu/L and HNO
(m):4, Pu valence:4). Test results were as follows. (1)Pu(IV) nitrate crystallization was not observed even in the case 200g Pu/L and HNO 6M and 4M which were considered to the best condition but crystal of H
6M and 4M which were considered to the best condition but crystal of H O and HNO
O and HNO
 3H
3H O were observed. (2)Similar results were obtained for the other parameter with lower Pu concentration. (3)We can estimate that Pu(IV) nitrate crystallization will not occurred in the reprocessing process with crystallization procedure. (4)The solubility data of Pu(NO
O were observed. (2)Similar results were obtained for the other parameter with lower Pu concentration. (3)We can estimate that Pu(IV) nitrate crystallization will not occurred in the reprocessing process with crystallization procedure. (4)The solubility data of Pu(NO )
) - HNO
- HNO -H
-H O system was obtained.
O system was obtained.
Tochiyama, Osamu*
JNC TJ8400 2000-044, 53 Pages, 2000/02
To estimate the polyelectrolyte effect and the effect of the heterogeneous composition of humic acids, the complex formation constants of Eu(III) and Ca(II) with Aldrich humic acid and polyacrylic acid were obtained, for Eu(10 to 10
to 10 M) by solvent extraction with TTA and TBP in xylene, for Ca (10
M) by solvent extraction with TTA and TBP in xylene, for Ca (10 M) with TTA and TOPO in cyclohexane and for Ca(10
M) with TTA and TOPO in cyclohexane and for Ca(10 M) by using ion-selective electrode. By defining the apparent formation as
M) by using ion-selective electrode. By defining the apparent formation as  = [MR
= [MR ]/([M][R]), where [R] denotes the concentration of dissociated functional group, [M] and [MR
]/([M][R]), where [R] denotes the concentration of dissociated functional group, [M] and [MR ] denote the concentration of free and bound metal ion and pcH is defined as-log[H], the values of log
] denote the concentration of free and bound metal ion and pcH is defined as-log[H], the values of log have been obtained at pcH 4.8 - 5.5 in 0.1 - 1.0M NaClO
have been obtained at pcH 4.8 - 5.5 in 0.1 - 1.0M NaClO and NaCl. Log
and NaCl. Log of Eu-humate varied from 5.0 to 9.3 and that of Ca-humate from 2.0 to 3.4..For both humate and polyacrylate, log
of Eu-humate varied from 5.0 to 9.3 and that of Ca-humate from 2.0 to 3.4..For both humate and polyacrylate, log increased with pcH or with the degree of dissociation. The increase in the ionic strength O.1 to 1.0 M decreased the log
increased with pcH or with the degree of dissociation. The increase in the ionic strength O.1 to 1.0 M decreased the log , the decrease in log
, the decrease in log of Eu(III)-humate is 1.6, that of Eu(III), polyacrylate 0.7, that of Ca(II)-humate 1.9 and that of Ca(II)-polyacrylate 1.2. While the increase in the metal ion produced no effect on log
of Eu(III)-humate is 1.6, that of Eu(III), polyacrylate 0.7, that of Ca(II)-humate 1.9 and that of Ca(II)-polyacrylate 1.2. While the increase in the metal ion produced no effect on log of polyacrylate, log
of polyacrylate, log of humate decreased. Depending on the concentration of Eu(III), the coexistence of Ca(II) reduced log
of humate decreased. Depending on the concentration of Eu(III), the coexistence of Ca(II) reduced log  of humate by 0 to 0.8. The dependence of log
of humate by 0 to 0.8. The dependence of log of humate on the metal ion concentration suggests the coexistence of strong and weak binding sites in the hmnic acid.
of humate on the metal ion concentration suggests the coexistence of strong and weak binding sites in the hmnic acid.
Amano, Hikaru; Ueno, Takashi; Arkhipov, N.*; Paskevich, S.*; Onuma, Yoshikazu*
Proceedings of 10th International Congress of the International Radiation Protection Association (IRPA-10) (CD-ROM), 6 Pages, 2000/00
no abstracts in English
Mihara, Morihiro; ; Ueta, Shinzo*; *
JNC TN8430 99-011, 27 Pages, 1999/11
In radioactive waste disposal, compacted Na-bentonite has been proposed for a buffer material. However, Na-bentonite would change to Ca-bentonite in the long term period. The change of Na-bentonite to Ca-bentonite might cause the change in the data concerning with nuclides migration properties such as permeability, sorption and diffusion. In this study, effective diffusion coefficients of HTO, Cs, I and C in compacted Ca-bentonite which was changed from Na-bentonite, Kunigel V1, were obtained and were compared to published those of Kunigel V1. In addition, effective diffusion coefficients for compacted Ca-bentonite with syncetic sea system water, SW, were obtained in order to investigate effect of solution composition. The magnitude of effective diffusion coefficients in Ca-bentonite are arranged in smaller order as Cs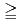 HTO
HTO I
I C. It is estimated that their effective diffusion coefficients are same those of Na-bentonite. About effect of solution composition, effective diffusion coefficients of HTO in 1.8g/cm
C. It is estimated that their effective diffusion coefficients are same those of Na-bentonite. About effect of solution composition, effective diffusion coefficients of HTO in 1.8g/cm dry density with SW were almost same values with distilled system water, DW. However, effective diffusion coefficients of HTO in lower density were smaller than values with DW. Regarding as effective diffusion coefficients of Cs in 1.8g/cm
dry density with SW were almost same values with distilled system water, DW. However, effective diffusion coefficients of HTO in lower density were smaller than values with DW. Regarding as effective diffusion coefficients of Cs in 1.8g/cm dry density, the effect of SW could not be observed as well as HTO. However, effective diffusion coefficients of I and C existing as an anion in pore water of bentonite increased by the reduction in the ion exclusion.
dry density, the effect of SW could not be observed as well as HTO. However, effective diffusion coefficients of I and C existing as an anion in pore water of bentonite increased by the reduction in the ion exclusion.
Sato, Haruo
JNC TN8400 99-060, 12 Pages, 1999/10
Apparent diffusion coefficients(Da) of Cs(Cs ), Ni(Ni
), Ni(Ni ) and Se(SeO
) and Se(SeO
 ) in a Na-bentonite (Kunigel-V1) were measured for a dry density of 1.8 Mg
) in a Na-bentonite (Kunigel-V1) were measured for a dry density of 1.8 Mg m
m with silica sand of 30 wt% at room temperature by in-diffusion method to evaluate the effect of the mixture of silica sand on Da in bentonite. The experiments for Cs and Ni were carried out under aerobic condition, but those for Se which is redox sensitive were carried out in an Ar glove-box (O
with silica sand of 30 wt% at room temperature by in-diffusion method to evaluate the effect of the mixture of silica sand on Da in bentonite. The experiments for Cs and Ni were carried out under aerobic condition, but those for Se which is redox sensitive were carried out in an Ar glove-box (O concentration
concentration  0.1 ppm). Consequently, no significant effect of silica sand mixture to the bentonite on Da values of Cs and Se was found, and the obtained Da values were approximately the same as those in the system without silica sand reported so far. On the other hand, Da values of Ni in bentonite with silica sand were 2 orders of magnitude lower than those in bentonite without silica sand obtained to date. The Da values of Ni reported so far were obtained using stable isotopic tracer and a tracer solution with fairly high Ni concentration compared with concentration used in this study was introduced. Additionally, it is known that distribution coefficient (Kd) of Ni on Na-montmorillonite which is the major constituent clay mineral of Kunigel-V1 decreases with increasing Ni concentration. Based on this, the abrupt decrease in Da values of Ni for bentonite with silica sand is considered to be due to the difference of sorption caused by the difference of Ni concentration in the porewater of bentonite.
0.1 ppm). Consequently, no significant effect of silica sand mixture to the bentonite on Da values of Cs and Se was found, and the obtained Da values were approximately the same as those in the system without silica sand reported so far. On the other hand, Da values of Ni in bentonite with silica sand were 2 orders of magnitude lower than those in bentonite without silica sand obtained to date. The Da values of Ni reported so far were obtained using stable isotopic tracer and a tracer solution with fairly high Ni concentration compared with concentration used in this study was introduced. Additionally, it is known that distribution coefficient (Kd) of Ni on Na-montmorillonite which is the major constituent clay mineral of Kunigel-V1 decreases with increasing Ni concentration. Based on this, the abrupt decrease in Da values of Ni for bentonite with silica sand is considered to be due to the difference of sorption caused by the difference of Ni concentration in the porewater of bentonite.
Saito, Junichi; Morinaga, Masahiko*; Kano, Shigeki
PNC TN9410 98-072, 97 Pages, 1998/07
Research on structural materials which will be utilized even in the severe environment of high-temperature liquid alkali metals has been promoted in order to develop the frontiers of materials techniques. The super-heat resisting alloys which are based on refractory metals, Nb and Mo, are aimed as promising materials used in such an environment. The corrosion resistance in liquid Li and the mechanical properties such as creep and tensile strengths at high temperatures are important for these structural materials. On the basis of many expeliments and analyses of these properties at 1473K, the material design of Nb-based and Mo-based alloys has-been carried out successfully. In this report, all the previous experimental results of corrosion tests in liquid Li were summarized systematically for Nb-based and Mo-based alloys. The corrosion mechanism was proposed on the basis of a series of analyses, in particular, focussing on the deposition mechanism of corrosion products on the surface and also on the initiation and growth mechanism of cracks on the corroded surface of Nb-based alloys. The principal results are as follows. (1)For the deposition mechanism, a reaction took place first between dissolved metallic elements and nitrogen which existed as an impurity in liquid Li and then corrosion products (nitrides) precipitated on the metal surface. Subsequently, another reaction took place between dissolved metalic elements in liquid Li, and corrosion products (intermetallic compounds) precipitated on the metal surface. The composition of deposited corrosion products could be predicted on the basis of the deposition mechanism. (2)For the crack initiation mechanism, the chemical potential diagrams were utilized in order to understand the formation of Li-M-O ternary oxides which caused cracks to be formed on the corroded surface. Consequently, it was evident that not only the concentration of the dissolved oxygen in the alloy but also the concentration of Li which ...
Kato, Toshihiro*; ; ; ; Ishibashi, Yuzo; Takeda, Seiichiro
PNC TN8410 98-070, 31 Pages, 1998/02
None
; ;
PNC TN9410 98-014, 46 Pages, 1997/11
Thermal conductivity of uranium-plutonium mixed oxide fuel for fast reactor at beginning-of-life was correlated based on the recent results in order to apply to the fuel design and the fuel performance analysis. A number of experimental results of unirradiated fuel speimens were corrected from open literatures and PNC internal reports and examined for the database. Thermal conductivity of acutual fuel with porosity ( ), that of fully dense fuel (
), that of fully dense fuel ( 100%TD) and porosity correction factor (F) had theoretically the following correlation :
100%TD) and porosity correction factor (F) had theoretically the following correlation :  = F
= F 100%TD. The following correlation was developed for fully dense fuel by the results of high density fuel pellets which the effect of porosity was relatively small. The data base ranged from 17 to 30% for plutonium content in heavy metal atoms, from 1.90 to 2.00 for oxygen to metal ratio, from 90 to 98% of theoretical density and from 400 to 2090 degree C for temperature.
100%TD. The following correlation was developed for fully dense fuel by the results of high density fuel pellets which the effect of porosity was relatively small. The data base ranged from 17 to 30% for plutonium content in heavy metal atoms, from 1.90 to 2.00 for oxygen to metal ratio, from 90 to 98% of theoretical density and from 400 to 2090 degree C for temperature. 
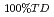 = (1/(-0.03237+0.8606
= (1/(-0.03237+0.8606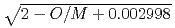 +2.483
+2.483 10
10 T))+75.27
T))+75.27 10
10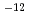 T
T where
where  100%TD: Thermal Conductivity (W/mK) T: Temperature (K) O/Z: Oxygen-to-metal ratio (-) In this work two porosity correction factors were needed for high density fuel and low density fuel (around the current Monju specification). For high density fuel (as-fabricated fuel density :
100%TD: Thermal Conductivity (W/mK) T: Temperature (K) O/Z: Oxygen-to-metal ratio (-) In this work two porosity correction factors were needed for high density fuel and low density fuel (around the current Monju specification). For high density fuel (as-fabricated fuel density :  90%TD) F
90%TD) F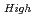 = 1-2.95P(P:Porosity volume Fraction (-)) For low density fuel (as-fabricated fuel density: around 85%TD) F
= 1-2.95P(P:Porosity volume Fraction (-)) For low density fuel (as-fabricated fuel density: around 85%TD) F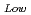 = 1-1.4P (P: Porosity volume Fraction (-)) The universal porosity correction factor was not determined in this work. In the next step, theoretical and analytical considerations should be taken into account.
= 1-1.4P (P: Porosity volume Fraction (-)) The universal porosity correction factor was not determined in this work. In the next step, theoretical and analytical considerations should be taken into account.